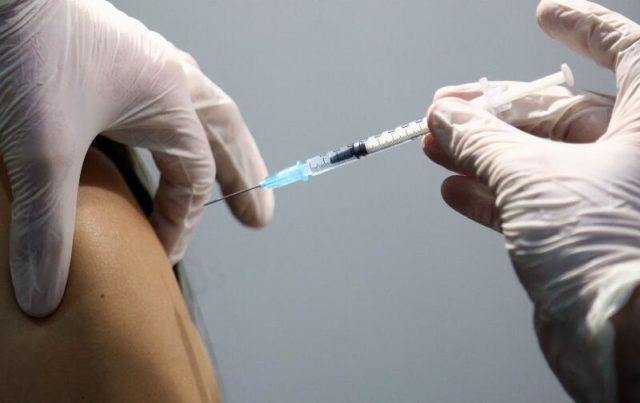
Last week, SA received one million doses of the Oxford-AstraZeneca vaccine, which is less effective against the Covid-19 variant that is dominant in South Africa
TRADE unions representing health care workers believe that the government’s “rushed” decision to procure the ineffective AstraZeneca vaccine gave rise to a series of blunders that have led to the suspension of the country’s vaccine roll-out programme.
Announcing the decision to halt the vaccine roll-out, Health Minister Dr Zweli Mkhize said further testing of the Oxford-AstraZeneca vaccine on the variant of Covid-19 dominant in South Africa revealed that it was less effective on mild and moderate infections.
Mkhize said more work would need to be done to map out a way forward for the vaccine and placed the planned roll-out on temporary hold.
Last week, SA received one million doses of the Oxford-AstraZeneca vaccine, which was subjected to testing ahead of the scheduled vaccination roll-out in mid-February.
The Democratic Nursing Organisation of SA (Denosa) said that before South Africa purchased more vaccines, the efficacy should be confirmed in the country first.
Denosa welcomed the decision to put the roll-out plan for AstraZeneca on hold.
“Denosa’s main concern is the safety of both health care workers and community members and, as such, acknowledges the superior scientific guidance that the government is getting and acting according to,” said the union.
The union also called for the speeding up of procuring the Johnson & Johnson and Pfizer vaccines and for the South African Health Products Regulatory Authority (Sahpra) to expedite their approval.
Lerato Mthunzi, the president of the Young Nurses Indaba Trade Union (YNITU), said the hopes of South Africans had again been dashed by a government “reckless” with making health care decisions.
Mthunzi said the union had warned about the rushed approach of procuring the Covid-19 vaccine without sufficient information and a clear strategy.
She said YNITU rejected the roll-out plan because the efficacy of the vaccine against the new strain of Covid-19 had not been proven.
“YNITU rejected these plans on the basis that there was no clear information as to whether the AstraZeneca vaccine would treat a new strain of virus that is currently prevalent in the country.”
Mthunzi said their members also raised concerns that they were expected to be first in line to receive the vaccine and later administer it to members of the public, but the Health Department was failing to provide clear information on the vaccine and its side-effects.
“The government’s rushed approach has resulted in taxpayers’ money being spent on a vaccine that is set to expire in April,” she said.
The union was also shocked that the government was so eager to procure a vaccine while failing to provide front-line workers with simple personal protective equipment.
The Health and Other Service Personnel Trade Union of SA (Hospersa) said it was disappointed that the vaccine would not be effective against the SA Covid-19 variant.
“This further puts more delays on providing sufficient protection to our members in the health sector. It further puts doubt in their minds about participating in the vaccine rollout campaign,” said Hospersa public relations officer Kevin Halama.
The union would continue to engage with the Department of Health to ensure that any vaccines made available to their members were safe for those who choose to take it in future and that those who choose not to be vaccinated would be afforded their rights, he said.
The Public Servants Association’s (PSA) assistant general manager, Reuben Maleka, said the union was extremely concerned about the pace of the vaccine roll-out.
“South Africa cannot afford to wait any longer for its citizens to be vaccinated and calls on government to go the extra mile to ensure the procurement of other available vaccines from other countries,” he said.
However, Maleka said it would serve no purpose to inoculate people with a vaccine that is not effective.
Maleka said the union was also concerned that the anticipated herd immunity would be even further delayed, which may result in unnecessary fatalities of front-line workers.
“This roll-out catastrophe will contribute to more doubts and fuel the negative narrative towards the vaccination in general, which must be guarded against at all costs,” he said.
The National Education, Health and Allied Workers’ Union (Nehawu) noted the positive results of the Johnson & Johnson vaccine which had over 44,000 trial participants.
“We call on our government to move with speed in procuring it because some of the participants were South Africans,” said Nehawu general secretary Zola Saphetha.
Nehawu also called for government to widen its net in searching for more vaccine options.
“We call on government to consider the Russian-made Sputnik V vaccine which published impressive phase 3 findings in the Lancet medical journal last week.”
Saphetha said the government should not confine its search for vaccines to Western and European manufacturers only.
He said vaccines produced by the Republic of Cuba’s Finlay Vaccine Institute was aimed at determining the optimal level of antigen strength for protecting people previously infected with Covid-19.
Health Minister Zweli Mkhize said the vaccination roll-out programme would proceed in the following manner:
– The programme for health care workers would be rolled out with the Johnson & Johnson and Pfizer vaccines together with an implementation study of AstraZeneca.
– The minister said AstraZeneca was purchased because the results at the time were known and it was available early.
– He said the hold on the vaccine was temporary until the issues were resolved.
– The Health Department is in discussions with the Serum Institute of India regarding the April vaccine expiry date for a possible extension or exchange of the vaccine.
– Shabir Madhi, professor of vaccinology at Wits University, was optimistic that in the next two months about three vaccines would come into clinical trials and would either be inclusive of the B1351 variant and the original variant or be able to adapt to the new variant.