SA health regulator recommends lifting pause on J&J vaccine roll-out
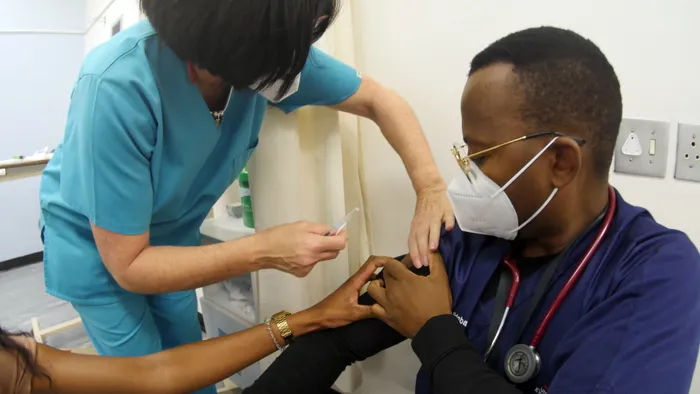
A medical front-line worker at Tygerberg Hospital receives a Johnson & Johnson Covid-19 vaccine jab before government paused the roll-out. Picture: Ian Landsberg/ African News Agency (ANA)
THE SOUTH African Health Products Regulatory Authority (Sahpra) has recommended that the government lift the pause on administering Johnson & Johnson's Covid-19 vaccines, given that certain conditions are met.
"These conditions include, but are not limited to, strengthened screening and monitoring of participants who are at high risk of a blood clotting disorder," Sahpra said on Saturday.
"In addition, measures are to be implemented to ensure the safe management of any participants who develop vaccine-induced thrombosis and thrombocytopenia (VITT)," the statement added.
Sahpra had said on Wednesday that it had recently reviewed data from Johnson & Johnson’s (J&J) local research study immunising health care workers and found no major safety concerns.
The country suspended the roll-out of the J&J vaccine in the "implementation study" on Tuesday, after US health agencies recommended pausing its use because of rare cases of blood clots in six people inoculated with it, out of some seven million people who have received the shot in the United States.
A US panel will meet again next week to discuss whether the pause on the use of the vaccine should continue, after delaying a vote on the matter earlier this week.
The Western Cape’s head of health, Dr Keith Cloete, said on Thursday that even though none of vaccinated health care workers using the J&J vaccine registered blood clots as side-effects, pausing the vaccinations was the ethical thing to do.
Speaking during a weekly digicon, Dr Cloete said: “We understand that such decisions have big implications for trust in the vaccine, the disruption of the programme but weighing all of these things up we have to understand the reason behind the pause.
“The big issue is that the J&J vaccine in this country has been made available via the Sahpra for trial conditions and as such we are bound by ethics to be cautious.
“When an event like the six clots in 6.6 million people was announced publicly by the FDA to say they are putting a pause to study the context of those cases our scientific community and the ethics committee here were bound by that decision because internationally the product we are using has been for trial conditions.”
- REUTERS
* Additional reporting by Mwangi Githahu